“FDA approves rapid diagnostic test for Candida auris”
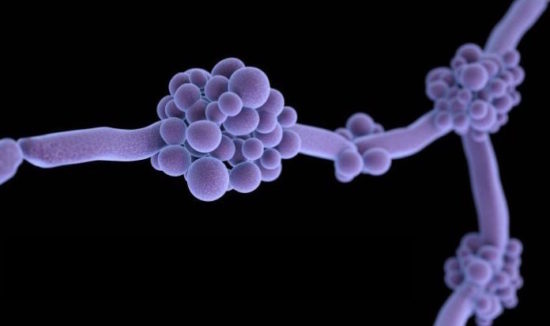
“The US Food and Drug Administration (FDA) has authorized the first rapid test to identify the emerging multidrug-resistant fungal pathogen Candida auris.
On Apr 20 the FDA announced that it was permitting the Bruker MALDI Biotyper CA System to be marketed for the identification of C auris, which first appeared in the United States in 2016 and to date has been detected in 287 US patients, with 257 cases confirmed, according to the Centers for Disease Control and Prevention (CDC).
The yeast causes severe infections in hospitalized patients, is associated with high mortality, and is difficult to identify with standard laboratory methods. Misidentification can lead to inappropriate management of infections and allow the pathogen to spread.”
Source: CIDRAP